- Department of Chemistry, IIT Hyderabad
- +91 (40) 2301 6015
- office@chy.iith.ac.in
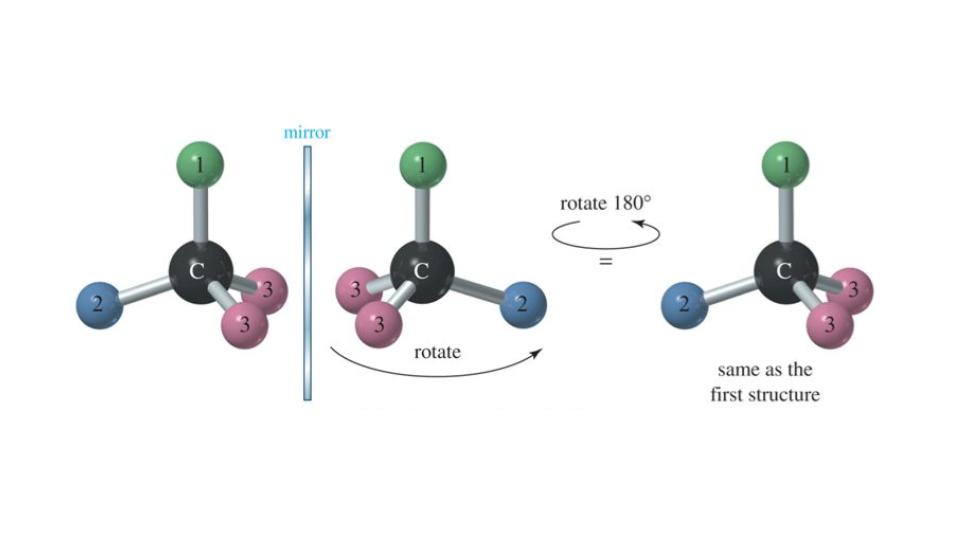
Stereochemistry: Introduction to molecular symmetry and point groups. Topicity and prostereoisomerism, nomenclature of stereotopic ligands and faces, stereoheterotopic ligands and NMR spectroscopy. Centre of chirality, assignment of absolute stereochemistry, CIP rules, axial chirality, planar chirality and helicity, descriptors for absolute stereochemistry. Conformational analysis: acyclic systems, cyclic systems,cyclohexane and decalins, conformation and reactivity with examples from molecular rearrangements, neighbouring group participation, elimination reactions, formation and cleavage of epoxides, quantitative correlation between conformation and reactivity, Winstein-Eliel equation, Curtin-Hammett principle. Stereoselectivity: Classification, terminology, principle of stereoselectivity, examples of diastereoselectivity and enantioselectivity including few examples from pericyclic reactions. Circular dichroism, ORD, cotton effect, application of ORD and CD in steriods, examples illustrating the usefulness of Cotton effect. Reaction mechanisms: Definition of reaction mechanism, transition state theory, kinetics, qualitative picture. Substituent effects, linear free energy relationships, Hammett equation and related modifications. Basic mechanistic concepts like kinetic vs thermodynamic control, Hammond postulate, Curtin-Hammett principle, isotope effects, general and specific acid-base catalysis, and nucleophilic catalysis. Nucleophilic substitution: various types, stability and reactivity of carbocations, nucleophilicity and basicity, neighbouring group participation and rearrangements, steric effects in substitution reactions, classical and non-classical carbocations. Rearrangements: neighboring group participation, ring expansion, carbocation, pinacol, dienone-phenol, benzilic, Favorskii, Baeyer-Villiger and Beckmann rearrangements.
Periodicity in Properties: Ionization potential, electron affinity, ionic radii and electronegativity. Chemical Bonding: Ionic solids, Born-Haber cycle, covalent bonds, dipole moment, resonance, hybridization, geometry and shape of simple molecules. Coordinate Bond, Hydrogen bond.Introduction to Transition Elements: oxidation states and their stabilities, colour (excluding the details of electronic transitions) and calculation of spin-only magnetic moment; Coordination compounds: nomenclature of mononuclear coordination compounds, cis-trans and ionisation isomerisms, hybridization and geometries of mononuclear coordination compounds (linear, tetrahedral, square planar and octahedral), acid-bases concepts, Isolation of Metals, Extraction of commonly occurring ores and minerals of iron, copper, zinc, silver and gold. Chemical principles and reactions of extractive metallurgy, Carbon reduction method (Iron), Self-reduction method (Copper), Cyanide process (silver and gold).
Basic electrochemistry (Nernst equation, concentration cells), Debye-Huckel theory, Debye-Huckel-Onsager equation, ion transport in solution: migration, convection and diffusion: Fick’s laws of diffusion, ion-solvent interactions, ion-ion interactions, electrode-electrolyte interface phenomena, problems based on diffusion and DH theory, the Helmholtz-Perrin, Guoy-Chapman and Stern models, polarization and overpotential, Butler-Volmer Equation, systems of technological interest (e.g. Electrolytes for Electrochemical Cells).
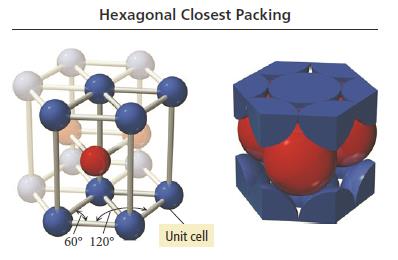
X-ray diffraction, principles of inorganic crystal structures, crystal chemistry and bonding in solids; preparative methods, characterization of inorganic solids: application of physical techniques, thermal analysis, electronic properties and band theory: metals, semiconductors, inorganic solids, colour, electrical, magnetic, thermal, and optical properties
Introduction: importance, historic background, quantum mechanics vs classical mechanics, Failure of classical mechanics, wave particle duality, uncertainty principle. Postulates, Schrödinger equation: wave function and interpretation, time dependent and time independent Schrödinger equation, eigenvalue problem. Quantum mechanics of some simple systems: free particle, particle in a box, harmonic oscillator, Angular Momentum: rigid rotor, orbital and spin angular momentum. Hydrogen and hydrogen like atoms, Approximate methods: perturbation theory, variational method, some simple examples. Many electron, atom: Pauli anti symmetry principle, Slater determinant, He atom, Li atom, Vibrational and Rotational spectroscopy. Reference Books: (1) I. N. Levine, Quantum Chemistry, (2) D. A. McQuarrie, Quantum Chemistry, (3) D. A. McQuarrie, J. D. Simon, Physical Chemistry: A molecular approach, (4) P. W. Atkins, Molecular Quantum Mechanics
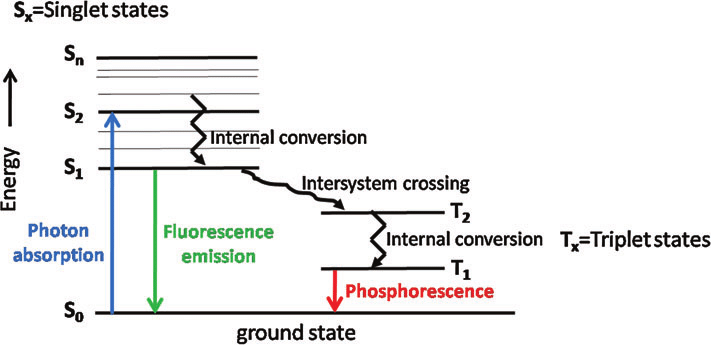
Surface Science: Interaction of radiation with matter, electronic transitions, Frank-condon principle, Jablonski diagram, basics of fluorescence (Stern Volmer equation, quenching etc), phosphorescence, delayed fluoresence (temperature effect, E and P, TADP), lifetime, applications of fluorescence, block diagram of spectrofluorometer, basics of photochemistry (with examples like C-H bond activation), photochemical reactions with examples, basics of lasers, theory of laser action, types of lasers (solid, liquid and gas, with detailed examples on their working), application of lasers, surface spectroscopy: XPS, AES and UPS. Types of statistics: Maxwell Boltzmann, Bose-Einstein, Fermi-Dirac statistics, Partition Functions: translational, rotational, vibrational, electronic and nuclear, Thermodynamics properties of monoatomic, diatomic gases considering distinguishable and indistinguishable particles, molar partition function of a system, partition function of a real gas, equilibrium constant of an ideal gas reaction, Einstein and Debye Theory of heat capacities, phase space, Ensembles, Entropy, Gibbs Paradox, Introduction to Quantum statistical mechanics.

Review of basic principles of quantum theory and atomic structure. Electronic structure of many electron atoms and variation principle. Electronic structure of diatomic molecules. Born-Oppenheimer approximation, H2+ ion, electronic term symbols, valence bond(VB) theory of diatomic molecules, comparison of VB and MO theories. Hartree-Fock theory of atoms and extension to molecules. Self Consistent Field (SCF) wavefunctions for diatomic molecules, configuration interaction(CI) wave functions. Electronic structures of polyatomic molecules. Basis functions. SCF-MO treatment of simple molecules, Koopmans theorems. Introduction to electron correlation. Mller-Plesset (MP) perturbation theory and CI calculations. Virial and Hellmann Feynman theorems. Huckel theory applied to conjugated molecules. Electron density theory, Semi-empirical methods, Molecular mechanics and Force fields, Operations and symmetry elements, The symmetry classification of molecules, Some immediate consequences of symmetry, Applications to molecular orbital theory and spectroscopy, Character tables and symmetry, labels, Vanishing integrals and orbital overlap, Vanishing integrals and selection rules.
Nuclear Magnetic Resonance Spectroscopy: NMR phenomenon, spin 1/2 nuclei, 1H, 13C, 19F and 31P, Zeeman splitting, Boltzmann distribution, effect of magnetic field strength on sensitivity and resolution. 1H-NMR, chemical shift, anisotropic effects, chemical and magnetic equivalence, coupling constants. Karplus relationship of J on dihedral angle, first order splitting patterns and structure correlation. Second order effects on the spectrum, AB, AMX, AA’BB’ spin systems, simplification of second order spectra. High field NMR, double irradiation, selective decoupling, chemical shift reagents. 13C satellites. - 13C-NMR, natural abundance, sensitivity. Introduction to FT technique, relaxation phenomena, NOE effects, 13C chemical shifts and structure correlations, off-resonance spectrum. - Dynamic processes by NMR, restricted rotation (DMF, DMA, biphenyls, annulenes), cyclohexane ring inversion, degenerate rearrangements (bullvalene and related systems), examples from few organometallic systems. Significance of coalescence temperature. - Introduction to 31P and 19F NMR. Infrared and Raman spectroscopy: Vibrational modes, group frequencies of organic, inorganic and organometallic systems, factors affecting the group frequencies, study of hydrogen bonding effects, vibrational spectra of ionic, coordination and metal carbonyl compounds. Mass spectrometry: Basic principle, ionization methods, isotope abundance, molecular ions, fragmentation processes of organic molecules and deduction of structural information, high resolution MS, introduction to soft ionization techniques and illustrative examples in macromolecular and supramolecular chemistry. Electronic spectroscopy: Electronic levels and types of electronic transitions in organic, inorganic and organometallic systems, solvent effects, effect of extended conjugation, Woodward-Fieser rules for calculation of absorption maximum, stereochemistry and electronic absorption.
Metal ions in biology: metallo-proteins and enzymes containing Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Mo and W ions. heme and non-heme systems, Peptide and nucleotide hydrolytic enzymes, Metal environment, electronic, magnetic and redox properties; fixation of N2, water-oxidation reactions, Synthetic models for the structure and function of the above enzymes, syntheses of ligand-metal complexes, reactivity of O2, CO, NO, N2; mechanistic aspects, high-valent metal-oxo (Fe-, Mn- and Cu) systems, Interaction of metal ions with nucleotides and peptides, hydrolysis of phosphate and amide groups, Metal based drugs, environmental applications and toxic effects.
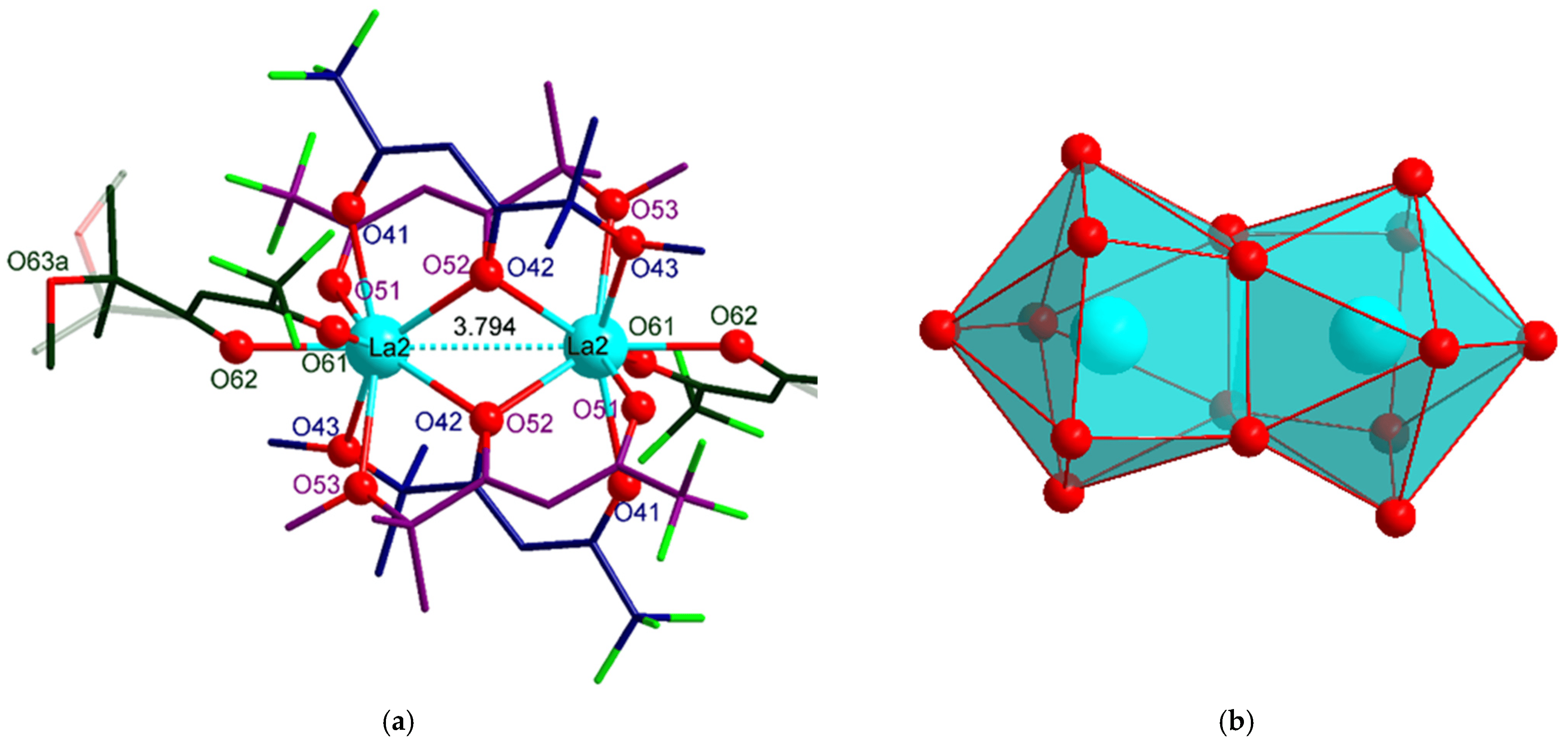
Course syllabus: Intrinsic Properties of the Lanthanide Elements (Electronic Features,
Steric Features). Synthesis of Organolanthanide Compounds (Thermodynamic and
Kinetic Guidelines, Inorganic Reagents, Metalorganic Reagents, Thermal Stability).
Ligand Concepts (Steric Bulk and Donor Functionalization, Ancillary Ligands,
Immobilization – “Supported Ligands”. Reactivity Pattern of Organolanthanide
Complexes (Donor-Acceptor Interactions, Complex Agglomerization, Ligand Exchange
and Redistribution Reactions, Insertion Reactions, Elimination Reactions– Ligand
Degradation, Redox Chemistry, Reaction Sequences – Catalytic Cycles, Side Reactions.
Lanthanide Triflate-Catalyzed Carbon-Carbon Bond-Forming Reactions in Organic
Synthesis. Lanthanide - and Group 3 Metallocene Catalysis in Small Molecule Synthesis.
Ref. Books. (1) Principles in Organolanthanide Chemistry, Reiner Anwander, Topics in
Organometallic Chemistry, Vol 2, Springer, Germany, 1999. (2) Lanthanide and Actinide Chemistry, Simon Cotton, John Wiley andamp; Sons Ltd, The Atrium, Southern Gate,
Chichester, England, 2 nd Ed, 2006.
Structure of TM complexes , ligands, hapticity, 18- electron rule, Clusters and M-M bonds, Reaction mechanisms, Metal alkyl and hydride, Metal-carbene complexes, Fischer/Schrock carbenes, NHC’s, olefin metathesis, multiple bonding between TMs and heteroatoms - p-complexes (olefins, dienes, alkynes, allyls, arenes) - Metallocenes - structures, syntheses, properties - OM complexes of alkali metals, Grignard reactions - Main group OM chemistry (group 13-16), Carbonylation of Alcohols- Hydrogenation of Alkenes- Hydroformylation - Alkene and Alkyne Metathesis. Oligomerization and Polymerization of Alkenes and Alkynes. C-C Coupling Reactions, C-Heteroatom Coupling: Aminationof Arenes, Hydroamination, Hydroboration, and Hydrosilation.

Brief overview of Electrochemical Techniques and their application to Real Systems, Electrochemical Cells: Batteries, Supercapacitors, Fuel Cells, Solid Electrolytes and Photoelectrochemical Cells (Dye Sensitized Solar Cells, Quantum Dots solar cells, Water Splitting), Perovskite Solar Cells, Photocatalysis, steam reforming, petroleum refining, coal reforming, hydrogen production, decomposition of N2O, dry reforming.
Introduction to Nanoscience: classification of nanomaterials - zero dimensional , one dimensional nanostructures - nanowires and nanorods, two dimensional nanostructures - films, nanotubes and biopolymers, three dimensional nanostructures - fullerenes and dendrimers, quantum dots and their properties, synthesis and application of nanomaterials-dye-sensitized solar cells, photocatalysis etc, basic instrumentation and imaging techniques. Intermolecular Interactions, Principles of self-assembly, supramolecular chemistry, soft lithography, nano/micro-contact printing-stamps and tips, layer by layer assembly, meso-structures from soft building blocks, nanocrystals-synthesis and self-assembly, templating methods, photonic crystals, nanorods-, nanotubes-, nanowires- self-assembly.